Car T Therapy Process

The process for car t cell therapy can take a few weeks.
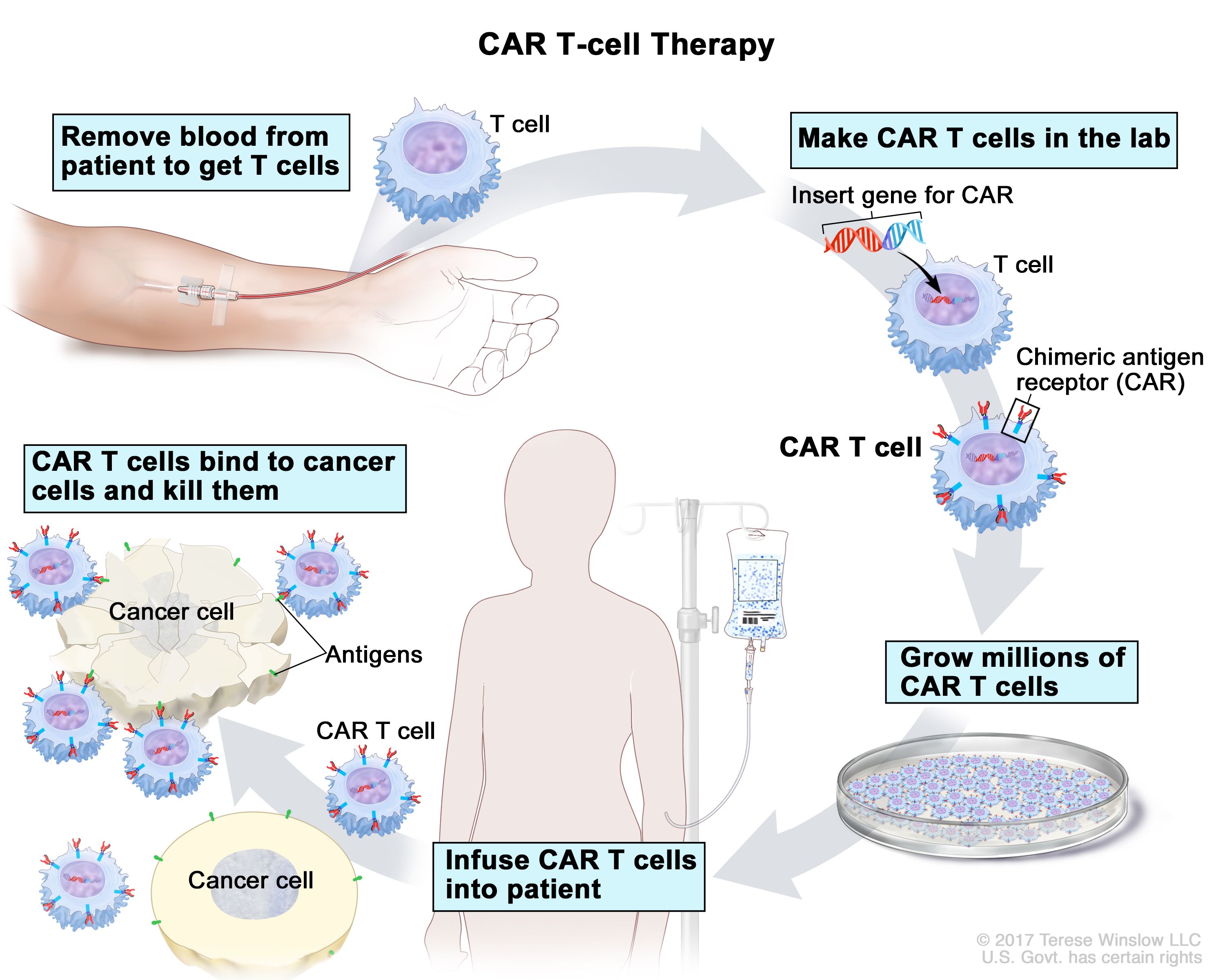
Car t therapy process. Two iv lines are needed because blood is removed through. T cells are collected from patients via apheresis a process that withdraws blood from the body and removes one or more blood components in this case t cells. 1 once an appropriate patient is identified for car t cell therapy the next step is to connect with an authorized treatment center to initiate the screening and enrollment process as appropriate. Car t cell therapy involves a biomedical engineering process.
First white blood cells which include t cells are removed from the patient s blood using a procedure called leukapheresis. During this process t cells are separated and removed from the blood and the remaining blood is returned to the body. The process to produce and deliver car t cell therapy is complex. Described toxicity of anti cd19 car t cell therapy 12.
The patient s t cells are harvested through apheresis in the hospital and separated from other blood components such as red blood cell platelets and monocytes the amounts of specific subsets of t cells such as cd4 and cd8 may be optimized. Your separated t cells are sent to a laboratory where a special receptor called a car or chimeric antigen receptor is added to them. However the optimal subsets of t cells to improve efficacy and limit toxic. Patients undergo a series of tests and screenings to determine if car t cell therapy is an appropriate treatment option.
Car t cell therapy is a complex multi step process for patients. In some studies up to 90 percent of children and adults with b all whose disease had either relapsed multiple times or failed to respond to standard therapies achieved remission after. The process begins by collecting blood from the patient with cancer. Chimeric antigen receptor t cell clinical trials have generated impressive results in the early outcomes of car t cell therapy patients with blood cancers.
Collecting the t cells. Doctors take a type of white blood cell from your body and genetically. Hospital settings for car t cell therapy production 24 box 6. The process of transforming your t cells into yescarta car t cells can take 2 3 weeks.
While ontario is building capacity for car t cell therapy the province can now treat a limited number of patients from ontario and other provinces and territories. How does car t cell therapy work. During this procedure patients usually lie in bed or sit in a reclining chair. This procedure is called leukapheresis or apheresis and is similar to the process of giving certain types of blood donations.
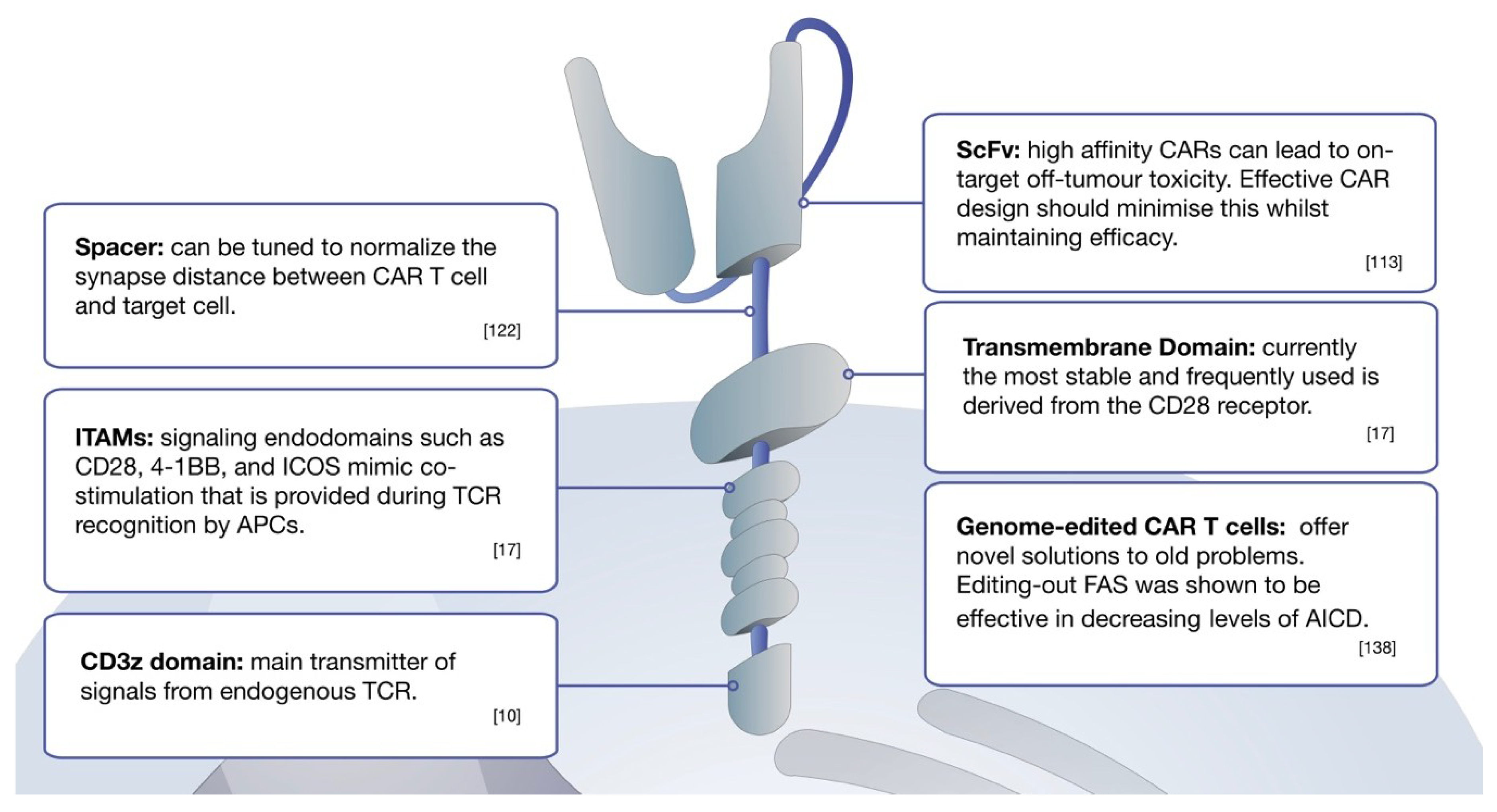
Chimeric antigen receptor car t cell therapy is a kind of cancer treatment that uses cells from your own immune system. This special connector improves your t cells ability to latch onto cancer cells and destroy them. Car t cell therapy process 8 figure 3. Structure of first second and third generation chimeric antigen receptors cars 7 figure 2.
Summary 26 figures figure 1. Availability of car t cell therapy in canada.