Car T Cell Therapy Mechanism Of Action

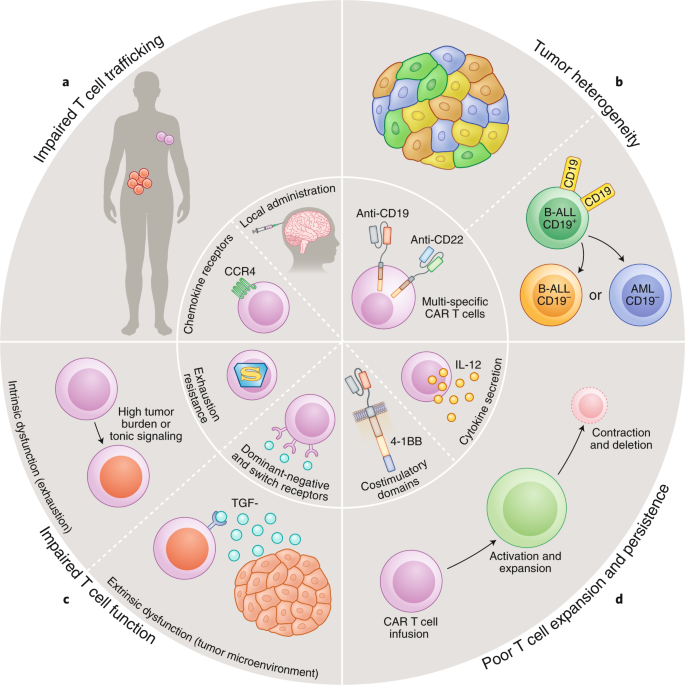
Given the extreme potency of car modified t cells the use of this therapy has significant toxic potential 94 96.
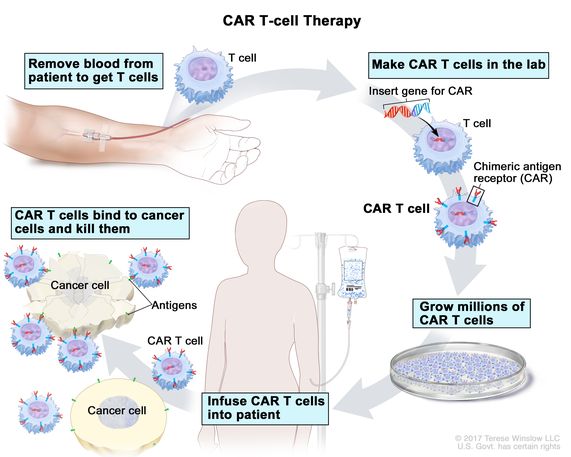
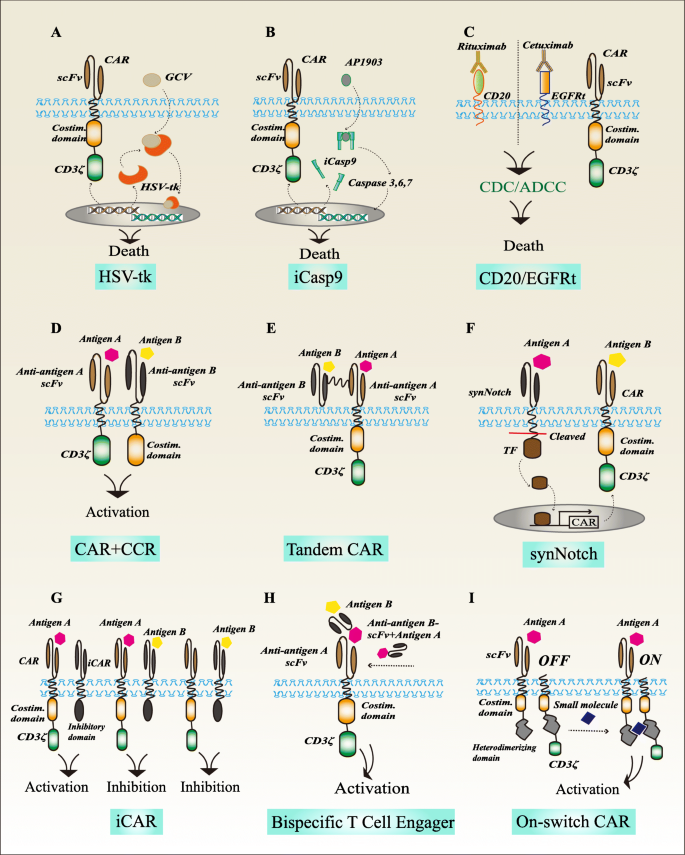
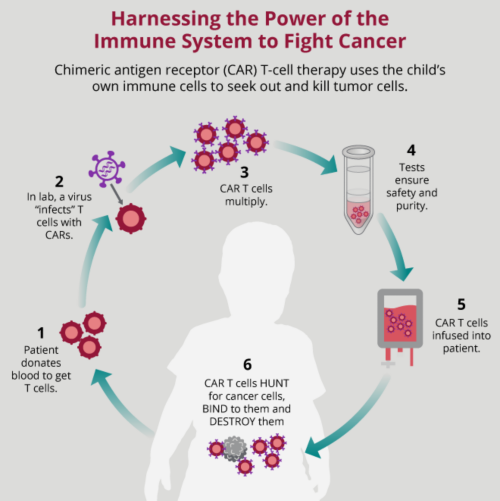
Car t cell therapy mechanism of action. Chimeric antigen receptor car t cell therapy is an investigational form of immunotherapy in fact the first gene therapy if fda approved. Kymriah is an individualized therapy that reprograms a patient s own t cells with a chimeric antigen receptor car containing a 4 1bb costimulatory domain. Overall it is clear that the potential of cd4 car t cells to mediate multiple target cell killing could further potentiate the efficacy of car t cell therapy. Factors affecting safety of car t cell therapy.
Car t cell therapy is a novel treatment approach. Toxicities range from life threatening cytokine release syndromes crs and macrophage activation syndromes mas to on target off tumor toxicity neurotoxicity and tumor lysis. Car t cell therapy is offering promising options for patients and clinicians who are struggling with refractory cancers that do not respond to or are ineligible for other standard options such as surgery. Normally t cells have a direct killing action on cancer cells and also communicate with the immune system via cytokines cell signalling molecules.
Car t cells are genetically modified t cells that express a car targeted against a specific taa which upon binding initiates t cell. The external targeting domain binds to the antigen activating the car t cell. Car t cell therapy works with the patient s own immune system to help boost the cancer killing effects of the t lymphocyte. Some examples include prior lines of therapy upper age limit severity of comorbidities and chemoresistance status.
The 4 1bb costimulatory domain is responsible for enhancing the expansion and persistence of kymriah. It is generally an autologous cell therapy that may have different patient selection considerations than autologous stem cell transplant asct. 4 5 eligibility for asct may differ from eligibility for car t cell therapy. Moving forward there is a need to identify and deliver optimal car t cell subset compositions an area which is already in focus for current clinical research 105.
Given the increasing use of car t cells as approved therapeutic agents and in clinical trials moffitt researchers believe that it is crucial to understand the mechanisms of how these therapies. Chimeric antigen receptor car modified t cell therapy.